COVID-19 update, Nov 21,2022
Many (if not most or even all) regions of Canada are facing significant pressures on the health care system due to increases in respiratory virus infections:
- COVID-19 continues to circulate at fairly high levels throughout the country. While there has not been a corresponding increase in hospitalizations or deaths (likely as a result of high levels of population immunity associated with vaccination and/or recent infection), emerging new strains (such as BQ.1 and BQ.1.1) could change this, as they are associated with even higher levels of transmissibility and immune escape compared to the currently predominant strain (BA.5).
- Influenza has reached epidemic levels earlier in the season than usual and case counts are quite high (in Canada, influenza is characterized as an epidemic when test positivity rates exceed 5%. This generally occurs every fall, but has not over the past two years presumably as a result of COVID-19 mitigations that were in place). As has always been the case with influenza, there is a higher risk of severe clinical outcomes (severe symptoms, hospitalization, death) associated with factors such as age, comorbidities, immunosuppression, and certain social/economic factors.
- RSV (Respiratory Syncytial Virus) infections have reached much higher levels than what has been the historical norm. RSV can infect people of all ages, and infection rates typically peak in the autumn. In most adults and older children, RSV infection results in cold/cough type symptoms. However, in younger children, more severe symptoms can arise such as croup/wheezing, which may lead to ER visits or hospitalization. Adults can transmit RSV to children, so this virus is a concern for the entire community. One theory for the significant increase in cases compared to the average is that for the last 2 fall seasons, there has been very little circulating RSV (again likely related to COVID-19 restrictions and mitigations in place), so several years’ worth of young children are being exposed to RSV all at the same time instead of having the same number of cases spread over several years).
The upswing in respiratory virus infections is also occurring in the context of other concerns that we have noted previously, including:
- Changes in public health guidance: As you know, Ontario (and other jurisdictions) have altered isolation requirements, raising concerns that individuals with infections are less likely to remain at home when sick and may be more likely to be present in the workplace or other public spaces (thereby increasing community infection transmission).
- Suboptimal vaccine uptake: The proportion of Canadians who have remained up to date with their COVID vaccinations has declined over time. This is a particular concern in light of the next point.
- Decreasing effectiveness of the immune response over time on a population level (as time since last vaccination and/or infection increases, the protection afforded against infection declines (however, the protection against severe outcomes decreases more slowly)).
Key Actions you can take:
- Stay up to date with your COVID-19 vaccinations. Generally (for most working age individuals), this means getting a booster if it has been more than 6 months since your last vaccine dose or infection. Attached is a flow chart that takes people through the decision making regarding timing of the booster.
- Get a flu shot. You can have the flu shot at the same time as your COVID booster.
- Stay home if you are ill, and consult employee health regarding when you can return to work. Continue to wear a well-fitted mask when you are inside around other people until at least 10 days have elapsed since the onset of your symptoms.
- Wearing a mask when in indoor public spaces (including the workplace) is strongly recommended to help protect you against infection with COVID, influenza, and other respiratory viruses. Wearing a mask will enhance your protection even if nobody else around you is wearing a mask.
- Wash or sanitize your hands before touching areas of your face and before eating. Hand hygiene can significantly reduce the risk of becoming infected with common respiratory and gastrointestinal viruses.
- If you are immunocompromised, have underlying health conditions, or are older, consult your health practitioner about whether available preventive medications(e.g., Evusheld) and treatments (e.g., Paxlovid, Tamiflu) are appropriate for you.
Update on November 16, 2022
Masks are strongly recommended for travelling or close contact indoors
Canada is currently experiencing an early surge of Respiratory Syncytial Virus (RSV), which has created record levels of pediatric hospitalizations for the virus. This influx coincides with the beginning of flu season and an increase in COVID-19 transmission creating a triple threat for our healthcare system. While it is a distracting conversation, particularly as we head towards winter and the holiday season:
IT IS STRONGLY RECOMMENDED TO WEAR A MASK WHEN WORKING OR TRAVELLING AND ESPECIALLY IN CLOSE PROXIMITY TO OTHERS.
Canada’s Chief Public Health Officer Theresa Tam shared that cases of RSV and influenza have now risen above seasonal levels. Federal data shows there are nearly 300 COVID-19 deaths per week, and more than 6,000 hospital beds are occupied by COVID-19 patients across Canada.
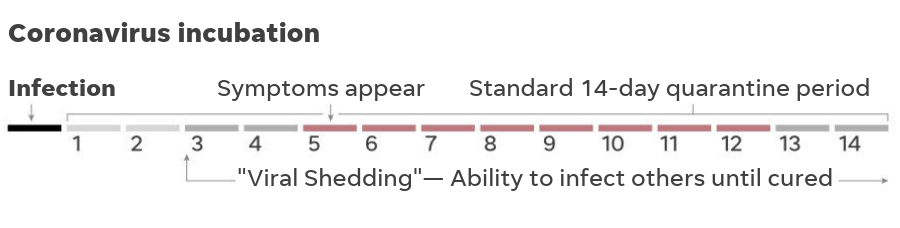
Please remember the incubation period for COVID-19 is typically much longer than RSV or influenza. Symptoms generally appear within three to seven days after exposure, but it may take as long as 14 days.
- Stay up to date with your COVID-19 vaccinations: Generally (for most working age individuals), this means getting a booster if it has been more than six months since your last vaccine dose or infection.
- Get a flu shot: You can have the flu shot at the same time as your COVID-19 booster.
- Stay home if you are ill: Consult Employee Health regarding when you can return to work. Once you return, continue to wear a well-fitted mask when you are inside and around other people for at least 10 days after the onset of your symptoms.
- Wear a mask: Wearing a mask is strongly recommended when indoors to help protect you against infection with COVID-19, influenza, and other respiratory viruses. Wearing a mask will enhance your protection even if nobody else around you is wearing a mask.
- Wash your hands: Washing or sanitizing your hands before touching your face and before eating is an effective precaution to limit the spread of viruses.
- If you are immunocompromised, have underlying health conditions, or are older, consult your health practitioner about whether available preventive medications and treatments are appropriate for you.
The attached infographic assists in determining when to get a COVID-19 Booster Shot.
For any questions, please contact Anthony, Dan or Trish.

Update on September 8, 2022
Update on January 19, 2022
Omicron Variant & Business Planning – January 11, 2022
Omicron Variant Q&A – January 10, 2022
COVID-19 FAQs as of December 17, 2021
There are two main elements that went into this change:
- In the context of the Delta variant, there was already evidence that immunity and vaccine effectiveness against infection was decreasing slowly after the initial 2 doses, so that by 6 months out, there was a meaningful impact on community infection rates. There is ample evidence that booster doses restore very high levels of immunity and vaccine effectiveness.
- With Omicron, the early data is demonstrating that 2 doses of mRNA or AstraZeneca vaccine are likely to offer very little protection against infection except in the first few months after immunization (when antibody levels are very high), but that high levels of protection are restored by administering a booster dose. Now that Omicron has emerged and is spreading rapidly, there is a pressing need to get boosters into as many arms as possible, as quickly as possible, to try to dampen the spread of Omicron. Waiting 6 months for a booster is no longer a viable option, because we need a higher wall to outpace the spread of Omicron, and in the latter part of the 6 months, people will have suboptimal protection.
Omicron Variant of Concern – December 9, 2021
COVID-19 FAQs as of November 23, 2021
COVID-19 FAQs as of November 1, 2021
Here is the latest information about COVID-19 and B&M activities and responses in the format of Frequently Asked Questions (FAQs). New questions and answers have been added below. You will also find some updated answers to previously submitted questions, shown in italics, in the Past FAQs section.
“Information on this page does not constitute medical advice or treatment recommendations. Please contact your health care provider if you have any concerns”.
Why You Should Vaccinate Your Kids Against COVID-19 – November 1, 2021
Booster vaccines for COVID-19 – November 1, 2021
Booster doses of the COVID-19 are being considered or offered in many jurisdictions. Booster doses are distinct from the “third doses” that have been available for several months for immunocompromised persons, who would have a suboptimal response to their initial vaccine series of two doses. Booster doses are intended to address the slow decrease in immunity that occurs over a period of months following vaccination.
Based on evidence to date, booster doses are safe and well tolerated. There is clear evidence that administration of a booster dose results in marked reductions in the risk of infection and severe illness, over and above the risk reduction resulting from the first two doses of the vaccine.
On 29 Oct. 2021, the National Advisory Committee on Immunization (NACI) updated their recommendations for booster doses as follows:
- Populations at highest risk of waning protection following their primary series and at highest risk of severe COVID-19 illness should be offered a booster dose of an mRNA COVID-19 vaccine at least 6 months after completing their primary series. These populations include:
- Adults living in long-term care or other congregate settings that provide care for seniors (as recommended by NACI on September 28, 2021)
- Adults 80 years of age and older
- Other key populations who may be at increased risk of lower protection over time since vaccination, increased risk of severe illness, or who are essential for maintaining health system capacity, may be offered a booster dose of an mRNA COVID-19 vaccine at least 6 months after completing their primary series. These populations include:
- Adults 70 to 79 years of age;
- People who received two doses of the AstraZeneca Vaxzevria/COVISHIELD vaccine or one dose of the Janssen vaccine;
- Adults in or from First Nations, Inuit and Métis communities; and
- Adults who are frontline healthcare workers who have direct in-person contact with patients and who were vaccinated with a very short interval.
The application of this guidance may differ between provinces. For example, on 26Oct2021, British Columbia announced availability of booster shots beginning on 1Nov2021 for seniors 70+ and Indigenous people 12+, residents in independent living facilities, home care recipients, health care workers who received their first two doses on a shortened schedule (less than 42 days between dose 1 and dose 2), and people who live in rural and remote Indigenous communities. Beginning in Jan 2022, everyone else in BC will be able to receive a booster dose 6-8 months after their second dose.
In the US, booster shots are available for:
- Individuals who received the Pfizer-BioNTech vaccine or Moderna vaccine, at least 6 months after the second dose:
- 65 years or older
- Age 18+ who live in long-term care settings
- Age 18+ who have underlying medical conditions
- Age 18+ who work or live in high-risk settings
- Individuals 18 years and older who received the Johnson & Johnson vaccine, at least 2 months after the second dose.
As more data is accumulated regarding the effectiveness of the vaccines over time at preventing infections and severe complications such as hospitalization or death, recommendations regarding booster doses may change to reflect the best available evidence. Stay tuned for more information!
COVID-19 vaccination for children – what we know so far – November 1, 2021
Vaccines for COVID-19 have already been approved for adolescents aged 12 and up, and Health Canada and the FDA are currently reviewing data from trials of the vaccine in children aged 5-11 years. Approval of the COVID-19 vaccines for children in this younger age group is anticipated to occur in both countries within the coming days to weeks.
Published data with regard to the Pfizer-BioNTech vaccine, which was studied in more than 1500 children 5-11 years of age. The vaccine dose used was 10 micrograms, which is one third of the dose used for adults. The data shows that the vaccine was well tolerated and produced an immune response and side effects comparable to those seen in a study of people ages 16 to 25. The most common reactions included fatigue, headache, muscle pain, and chills. No cases of myocarditis/pericarditis were observed during the vaccination period through approximately 3 months of follow-up. Vaccine effectiveness against laboratory-confirmed symptomatic COVID-19 occurring at least 7 days after Dose 2 was approximately 91%.
Why should children receive the COVID-19 vaccine?
Until recently, the COVID-19 infection rate in children has been relatively low, and most infected children experience no symptoms or mild symptoms. However, some children do develop severe disease and might require hospitalization. In Canada, cumulative data to October 1, 2021 indicated that children aged 0 to 19 years old accounted for 20% of cases but much lower proportions of hospitalizations (2.0%), intensive care unit (ICU) admissions (1.2%), and deaths (0.1%%). Unfortunately, in recent months, the proportion of children experiencing COVID-19 infection has increased. In addition to acute COVID-19, children may develop a rare post-SARS CoV-2 complication known as multisystem inflammatory syndrome in children (MIS-C).
Children with certain underlying chronic medical conditions are at increased risk for severe COVID-19. It is reported that 39% of children admitted to hospital because of COVID-19 had at least one underlying comorbidity, most commonly chronic encephalopathy, obesity, asthma, chronic lung disease other than asthma, epilepsy, and neurodevelopmental disorders. Obesity, chronic neurological conditions, and chronic lung diseases other than asthma have been associated with greater COVID-19 severity. Other Canadian surveillance data indicate that the proportion of COVID-19 cases being hospitalized or admitted to an ICU is 4 to 5 times higher for individuals 12 years of age and older with immunodeficiency, than for the general population.
Along with the risks to physical health posed by COVID infection, the COVID-19 pandemic and the public health response to it have had significant indirect effects on children’s health. Disruptions in family routines, school and other educational activities, play, and sports, as well as separation from friends, grandparents, and other close family members, are affecting the mental health of children and adolescents in Canada, as manifested by eating disorders, anxiety, depression and problematic substance use.
Children can also transmit SARS-CoV-2 infection to others, which may pose a risk to adults in their family and community, especially if the adults themselves have underlying health conditions or are elderly.
Vaccination of children against COVID-19 will reduce the risk of infection and complications related to infection in children, and will reduce the risk that a child will transmit infection to adults around them. It is a vital step in helping children and their families “return to normal”, and will help to make our communities safer for everyone. Evidence to date shows that the COVID vaccine is safe and effective in children, and is associated with mild and relatively minor side effects.
Booster Vaccines – October 7, 2021
Third doses of the COVID-19 vaccine are being administered to different subgroups of people in many countries around the world. The evidence to date supports that third doses of the mRNA vaccines are safe and effective at increasing antibody levels, at least over the short term. To date, additional doses given have used existing vaccines rather than reformulated vaccines (there are several reformulated vaccines currently under study, in an effort to improve the immune response to emerging SARS-CoV-2 variants).
The decision to provide booster doses has been based on the observation that over time there has been a gradual decrease in protection against symptomatic infection afforded by the COVID-19 vaccine. This has been correlated with a gradual increase in infection rates among individuals who are fully vaccinated. This effect has been more pronounced in certain groups, including those who are older in age and those who are immunocompromised.
It is important to note that protection against severe COVID-19—including severe symptoms, hospitalization and death—from the two dose mRNA vaccines or one dose in the case of the Johnson & Johnson vaccine has not significantly decreased over time, and remains very high for the mRNA vaccines.
In Canada, the National Advisory Committee on Immunizations (NACI), an independent group of experts that advises Health Canada, has recently recommended that booster doses of an mRNA vaccine be offered to all long-term care residents and seniors living in other congregate settings, at an interval of at least six months after the primary series has been completed. Additionally, several provinces have begun administering booster doses to higher risk groups, with variation between provinces. For example, in Ontario, booster doses are offered at least two months after the primary series to:
- Transplant recipients, including solid organ transplant and hematopoietic stem cell transplants
- Those undergoing active treatment for solid tumors
- Individuals receiving therapy with an anti-CD20 agent commonly used for conditions such as multiple sclerosis, rheumatoid arthritis, leukemias/lymphoma, etc.
- Those undergoing active treatment with the following categories of immunosuppressive therapies: anti-B cell therapies (monoclonal antibodies targeting CD19, CD20 and CD22), high-dose systemic corticosteroids, alkylating agents, antimetabolites, or Tumor-Necrosis Factor (TNF) inhibitors and other biologic agents that are significantly immunosuppressive
- Individuals receiving active treatment for malignant hematologic disorders, including chemotherapy, targeted therapies, immunotherapy for AML, CML, ALL, CLL, etc.
- Those in receipt of Chimeric Antigen Receptor (CAR)-T-cell
- Those with moderate or severe primary immunodeficiency, such as DiGeorge syndrome and Wiskott-Aldrich syndrome
- Stage 3 or advanced untreated HIV infection and those with AIDS
In the USA, the Food and Drug Administration (FDA) recently authorized booster doses for the Pfizer-BioNTech vaccine for:
- Individuals 65 years of age and older
- Individuals 18-64 years of age at high risk of severe COVID-19
- Individuals 18-64 years of age whose frequent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19, including severe COVID-19
As new research emerges regarding the effectiveness of the COVID-19 vaccines over time and impact of booster doses, it is anticipated that the recommendations regarding receiving extra vaccine doses will change to reflect the growing scientific understanding.
Vaccine names
Coinciding with the granting of full authorization by the US Food and Drug Administration (FDA) and by Health Canada, the vaccine manufacturers are now legally allowed to market the vaccines using brand names. It is important to note that there has been no change in the vaccines themselves, and that the only change is to the label, which now incorporates a brand name in addition to the generic name.
The vaccine brand names are as follows:
- Pfizer-BioNTech vaccine: Comirnaty
- Moderna vaccine: Spikevax
- AstraZeneca vaccine: Vaxzevria, Covishield
- Johnson & Johnson vaccine: no brand name to date
Vaccine passports
Over the past several months, every Canadian province has announced plans to implement proof of vaccination programs, otherwise known as “vaccine passports”. This program has already come into effect across much of the country. While there are some differences in the precise implementation between provinces, it is generally the case that all individuals 12 years and older will be required to provide proof of vaccination to access non-essential settings (e.g., restaurants, clubs, sports venues, movies, gyms), but such proof would not be required for access to essential services (e.g., grocery stores, religious settings, personal service facilities). Proof of vaccination may be an electronic credential or a paper copy of a vaccination receipt (depending on the province and the stage of implementation). The programs generally provide an exemption for individuals who are medically unable to receive the COVID-19 vaccine. Since unvaccinated individuals are responsible for a disproportionate amount of the spread of COVID-19 at this time, implementation of these programs will help to reduce the risk of transmission in settings where proof of vaccination is required.
Mu variant
The Mu variant is a strain of SARS-CoV-2 that was first identified in Colombia in early 2021. Since then it has spread to many countries around the globe. The Mu variant has been classified as a “variant of interest” by the World Health Organization, because it has mutations that are associated with the potential for reduced vaccine effectiveness—there has been evidence that antibodies formed against other SARS-CoV-2 strains may be less able to neutralize the Mu variant. Despite the concern about this variant, it is important to note that apart from Colombia and Ecuador, the prevalence of the Mu variant remains very low (i.e., less than 0.5 per cent of cases in Canada). The Delta variant remains the predominant strain across both Canada and the U.S. at this time.
CDC (US) Information for Booster Shots – Sept. 28, 2021
Dr. Levine Q&A – Sept. 9, 2021
CBO – COVID-19 e-Q&A with Dr. Levine
Master Question Bank (July 27, 2021)
Summary of estimated vaccine efficacy (June 21, 2021)
A recent update from the Ontario Ministry of Health provided the following summary of estimated vaccine efficacy for the COVID-19 vaccines currently authorized for use in Canada, based on published evidence to date.
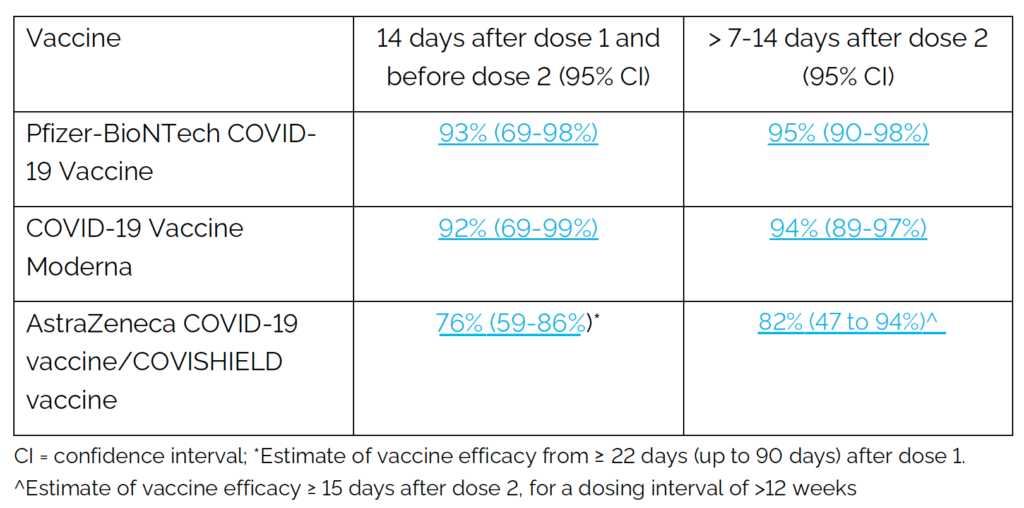
With regard to whether the effectiveness changes if one receives a different vaccine for the second dose, we are still awaiting evidence regarding the effect of mixed vaccination regimens on both immune response and risk of infection from an ongoing trial currently being conducted in the UK. This data is anticipated to be released by the end of June 2021. There is data available from smaller studies in Spain and Germany that suggests that mixing different vaccines might produce an improved immune response.
On June 17, 2021, NACI (the National Advisory Committee on Immunizations, an independent group of experts which advises Health Canada) released new guidance which included the following statement: “An mRNA vaccine is now preferred as the second dose for individuals who received a first dose of the AstraZeneca/COVISHIELD vaccine, based on emerging evidence of a potentially better immune response from this mixed vaccine schedule and to mitigate the potential risk of VITT associated with viral vector vaccines. People who received two doses of AstraZeneca/COVISHIELD vaccine can rest assured that the vaccine provides good protection against infection and very good protection against severe disease and hospitalization.”
Stay tuned for more information on this important topic as more evidence emerges!